in cooperation with
 |
| ... will be updated weekly... |
👉SLQ Consultants: Contact our experts HERE
The Medical Device Regulation (MDR)
The Medical
Device Regulation defines a medical device as “any instrument,
apparatus, appliance, software, implant, reagent, material or other article
intended by the manufacturer to be used, alone or in combination, for human
beings for one or more of the following specific medical purposes:
- diagnosis, prevention, monitoring, prediction, prognosis, treatment or alleviation of disease,
- diagnosis, monitoring, treatment, alleviation of, or compensation for, an injury or disability,
- investigation, replacement or modification of the anatomy or of a physiological or pathological process or state,
- providing information by means of in vitro examination of specimens derived from the human body, including organ, blood and tissue donations.
and which does
not achieve its principal intended action by pharmacological, immunological or
metabolic means, in or on the human body, but which may be assisted in its
function by such means. MDR also includes devices for the control or support of
conception; products specifically intended for the cleaning, disinfection or
sterilization […] Software shall also be deemed to be an active device.
Please click: You will find the document here.
Regulatory is still in the transition period. For the time being the Medical Device Directives with their national laws are still in place.
Standards and norms help you to be compliant with the medical device regulations (MDR). The most important standard for a medical device company is standard ISO 13485:
About ISO 13485
ISO 13485 process approach
treats the QMS as a set of interrelated processes covering not only the
manufacture of a product or provision of a service, but also management
processes and support processes. A "process" is something that
transforms a collection of inputs into outputs. Inputs consist of everything
needed to accomplish this transformation. For manufacturing a device these this
might include such things as raw materials, manufacturing supplies, work
benches, cleaning materials, tools, and equipment, the building, people,
written instructions, assembly drawings, comparison samples, and workmanship
standards, working instructions, test specifications. The output of the
process, that is the transformation of these inputs, produces the finished
part, records about what was done by who, and information about how the
transformation was accomplished, such as time to complete or production yield.
Unwanted outputs might include scrap parts and wasted material. For
non-manufacturing processes, for example document control, inputs might include
document control procedure, change request, people, equipment (copy machine,
computer, scanner), document control center, and the outputs would include
controlled documents, controlled copies, and process statistics. As you can see
from even just these two examples, the output of one process, i.e. document
control, is the input to other processes, such as manufacturing.
Of course there are more standards and norms applying to the design and the development of your medical device. The picture below should guide you through this jungle of norms and standards.
Of course there are more standards and norms applying to the design and the development of your medical device. The picture below should guide you through this jungle of norms and standards.
The lifecycle of a medical device
The lifecycle of a medical device can be divided in seven main stages "from birth to death"
During the different stages, various stakeholders are involved in realizing, distributing, and using a medical device.
The importance of a regulatory strategy
Planning regulatory submission activities from the very first beginning is essential for a successful approval of your medical device. Following this roadmap may be of assistance. Visualize your regulatory strategy and realize regulatory as a strategic planning game: There will be only one winner at the end of the game. It is YOU and your company. Use regulatory as helpful tool and it will guide you through your development process...
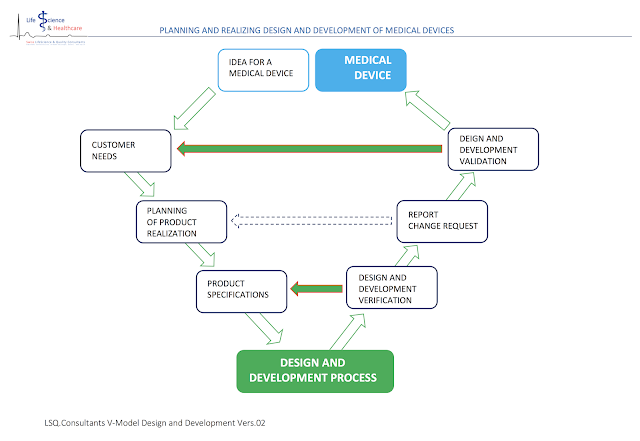
Planning and realizing design and development of medical devices
Every manufacturer of medical devices must document and record all design and development results in the form of specifications, manufacturing procedures, technical drawings and/or laboratory journals.
The results must be
available and be prepared in such a way that they can be verified against the
defined design and development requirements. All results must be released and
approved before the verification. The
manufacturer shall provide information for the procurement, production and service
activities resulting from the development results and establishing acceptance
criteria for the product.
1. THE IDEA FOR A MEDICAL DEVICE. Customer requirements and user needs:
At the very first beginning of your design and development work, listen to your customers needs: This can be a person (surgeon, patient, other user) but this can also be an analysis of the needs of the market.
Record and file all your findings. These findings are the very first specifications of your device. Bring them to paper and try to translate the more descriptive user needs into technical specifications of your device. Start a table and fill in [a] user needs, [b] the translation into technical specifications, [c] applying standards and norms, and [d] possible risks you can identify with the use of your medical device. Risks for the patient AND the user.
2. PRODUCT REALIZATION. Design and development planning:
In this chapter, you will learn more about the different design and
development phases and how they interact and build on one another. This chapter
is very important for you as developer and manufacturer, since designing a
medical device is a sequential process with given and defined design and
development phases that must be followed without any gaps.
A medical device
company that plans its product realization must plan and control the design and
development of (the) product. […] documents shall be maintained and updated as
the design and development progresses [ISO 13485:2016,
7.3.2]. The company must coordinate the different groups and experts that are
involved in this process.
Ask yourself these important questions:
Which processes (internal and external/outsourced) are
required for the design and development of compliant products? Which activities
and tasks must be performed to ensure those processes? What are the key
positions to be occupied? What other positions are required? What are the responsibilities
and authorities for the positions identified? Which knowledge, skills and
abilities are demanded for those roles, tasks and activities?
To answer these
questions, build up your processes in simple process maps. This helps you to
get an overview and visualization of all relevant processes and how they
correlate to each other, showing important interfaces and critical points
between the sequence of processes.
>> ISO 13485:2016 and MDR: Design and Development Documentation Requirements <<  |
| Please check this LINK: Product Realization - Documentation Requirements |












Keine Kommentare:
Kommentar veröffentlichen